International Statistic of the Year: Race for a COVID-19 vaccine
Tips
Liberty Vittert, Washington University in St Louis

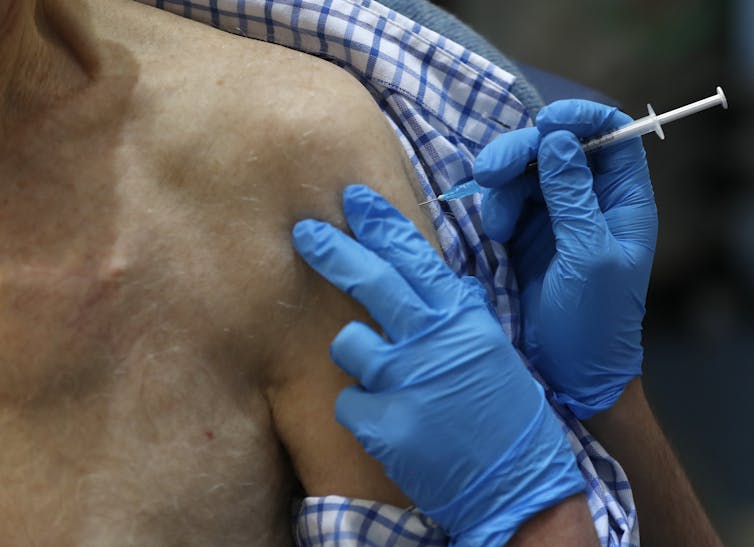
Scientists in China published the complete genetic sequence of SARS-CoV-2 on Jan. 10, 2020. On Dec. 8, 2020, health officials in London began administering an effective coronavirus vaccine to the public. The global scientific community successfully developed a COVID-19 vaccine in just 332 days.
I am a statistician, and this year I was on the judging panel for the Royal Statistical Society’s International Statistic of the Year. Much like Oxford English Dictionary’s “Word of the Year” competition, we choose one statistic that is meant to capture the zeitgeist of the year.
The statistic 332 days was the clear, standout winner. After a year of terrible tragedy, economic hardship and sorrow, this number represents an unparalleled collaboration in the history of medicine that gives hope for a return to normality in 2021.
Fastest vaccine development ever
In 1981, researchers established the link between human papillomavirus and cervical cancer, a disease that still causes hundreds of thousands of deaths per year worldwide. But it wasn’t until 2006, over 25 years later, that the first HPV vaccine was developed in the U.S.
On average, it takes over 10 years to develop a vaccine. Prior to this year, the fastest vaccine development was for the mumps vaccine. That took four years.
In April 2020, The New York Times played out multiple scenarios with vaccine experts as to how long it would take to get a SARS-CoV-2 vaccine. Under normal circumstances, experts estimated, a vaccine would be ready by November 2033.
So how is it possible that researchers got a vaccine to market in just 332 days?

Government financial investment
A number of things helped to get this vaccine done fast, including international collaboration of unseen proportions, an expedited trial phase process and the biology of the virus itself. In addition to these efforts, one very important reason for the incredible speed was the huge amount of investment made by governments at the very beginning of the pandemic.
Usually, pharmaceutical companies have limited resources they are willing to spend on vaccine development, and usually, governments are not willing to sink endless money into a process that they aren’t sure is going to work.
The COVID-19 pandemic has thrown the entire playbook to the wind. As of writing this, 641 therapies and 189 vaccines related to COVID-19 are under development, most of which are government-funded.
The U.S. government invested in multiple vaccines with the understanding that some of them wouldn’t work, but with the hope that a few would. Under Operation Warp Speed, the U.S. government quickly pledged almost US$9 billion to fund vaccine development and production. Moderna – whose vaccine is expected to become the second authorized for U.S. use after Pfizer’s, received a little under $1 billion of federal funding, with a further $1.5 billion for 100 million doses. While this number alone is not surprising – vaccines tend to cost between $521 million and $2.1 billion to develop – this was just one of many expensive projects.
Funding from governments and private donors also went toward building manufacturing facilities with the assumption that a vaccine was imminent and the usual regulatory hurdles would be passed quickly. For example, the Bill and Melinda Gates Foundation helped fund seven factories back in April, even though only one or two of the factories will actually be used.
This influx of money into multiple vaccines and early preparation for manufacturing was instrumental toward getting a vaccine developed and distributed in record time. The development of COVID-19 vaccines is a testament to the ingenuity, dedication and collaborative efforts of the scientific community. At the end of a seemingly hopeless year, there is a light at the end of the tunnel.
[Get facts about the coronavirus and the latest research. Sign up for The Conversation’s newsletter.]![]()
Liberty Vittert, Professor of the Practice of Data Science, Washington University in St Louis
This article is republished from The Conversation under a Creative Commons license. Read the original article.
The views and opinions expressed in the article are solely those of their authors, and do not necessarily reflect the opinions and beliefs of UDiversity.com.





Comments (5)
Best Company Reply
- Ali Tufan
- 2 days ago
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Fusce vel augue eget quam fermentum sodales. Aliquam vel congue sapien, quis mollis quam.Because Other Candidates
- Martha Griffin
- 23 August 2018
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Fusce vel augue eget quam fermentum sodales. Aliquam vel congue sapien, quis.Aldus PageMaker including versions Reply
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Fusce vel augue eget quam fermentum sodales. Aliquam vel congue sapien, quis mollis quam.